Advantage and Core Benefit
- Long-term sustained release of antibody drugs over 3 months
- Can be refilled with drug or gas once attached.
Background and Technology
The eye is an organ that is difficult for drugs such as eye drops and oral medications to reach the intraocular space due to the presence of a barrier function such as collagen. For this reason, the mainstream treatment for age-related macular degeneration and other diseases of the retina and choroid is direct intravitreal injection of antibody drugs such as anti-VEGF drugs. In addition, intravitreal injections need to be repeated every month to maintain the efficacy of antibody drugs, but there are concerns about complications such as intraocular inflammation caused by bacterial infection. In addition, it is often difficult for elderly patients to visit the hospital and continue treatment.
Although development is underway to prolong the efficacy of VEGF antibodies through formulation innovations, multiple intraocular injections are unavoidable, and implantable devices capable of sustained release for several months or longer with a single dose are expected to be developed. Existing technologies include reservoir-type devices in which drugs are stored in non-degradable polymers such as Vitrasert and Iluvien, and monolithic devices in which drugs are encapsulated in degradable polymers such as Ozurdex. However, each of these devices has problems in both sustained release and long-term retention of water-soluble drugs, making it difficult to apply them to antibody drugs.
Dr. Yasukawa and colleagues have developed a gas-filled hollow intraocular sustained-release device that utilizes the phenomenon of gradual absorption of antibody drugs according to the aqueous solubility of gas. In this device, a freeze-dried antibody drug is placed inside a hollow device, and through an open tip implanted in the eye, the gas inside the device is replaced by intraocular fluid, causing the antibody drug inside the device to be eluted.
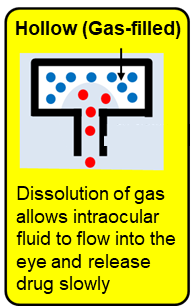 |
Data
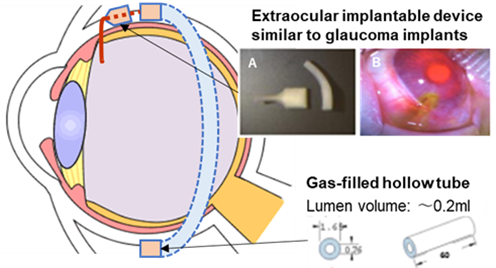
- 3D-printed extraocular implantable device (Upper figure A) and its actual implantation outside the animal’s eye (Upper figure B)
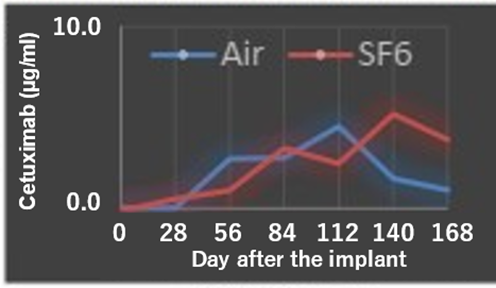
- Changes in antibody concentration in the anterior chamber after cetuximab was lyophilized in the device and filled with air or sulfur hexafluoride (SF6) gas and implanted in the rabbit eye (Lower figure)
- Drug dose can also be increased by connecting a hollow tube to the device (Upper panel)
 |
 |
Patent & Publication
US 11219598 (Grated), EP3453366
Researcher
Tsutomu Yasukawa (Nagoya City University)
Current Stage and Expectations
The concept and prototype have been completed, and we would like to work on increasing the drug dose and improving the sustained release by improving the material of the tube. We are seeking a company that is willing to co-develop an intraocular sustained release device based on this invention.
Project No.WL-04149a


