Advantage and Core Benefit
- Much higher sensitivity and specificity than CA19-9 or CEA confirmed in a retrospective study of patients with early-stage gastric cancer.
- Value increases in many solid tumors: esophageal, liver, pancreatic, colon, breast cancer.
Background and Technology
Barium test, fecal occult blood test, and blood tests for CA19-9 and CEA are performed as gastrointestinal tract examinations. The sensitivity of barium and blood tests to early-stage cancer are low, and the tumor detection rate is low in endoscopic examinations in fecal occult blood test positive subjects.
SDF4, which was identified from clinical samples and global database, is an ultrasensitive and specific marker with elevated blood levels in major solid tumors such as gastric, colorectal, and breast cancer, and can be detectable by ELISA, which may replace prevailing tests.
Data
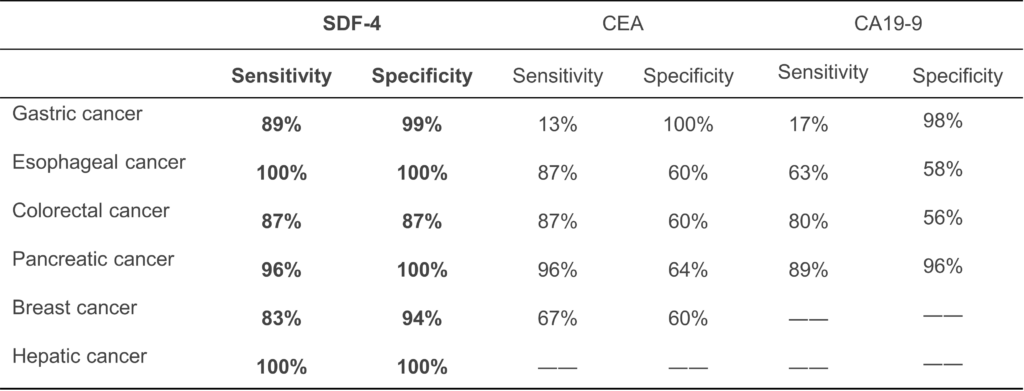 |
Patent, Publications
Patent pending (not yet published)
Journal: Scientific Reports
Researcher
Dr. Mitsuro kanda, Department of Gastroenterological Surgery, Nagoya University, Japan.
Expectations
An international prospective observational study for gastric cancer is being prepared (to be started in 2024). The researcher is working to obtain mAbs for ELISA kit.
We are seeking a IVD company who will develop the technology.
Project No. KJ-04009


