Advantages
- The efficiency of organic solvent extraction was improved by applying the molecular crowding effect, in which the equilibrium constants and reaction rates of biomolecules increase or decrease when the intracellular environment is crowded.
- It has been demonstrated that the addition of molecular crowding agents such as dextran to the aqueous phase increased complexation in the organic phase and promoted the efficiency of solvent extraction.
- This technology will contribute to reducing environmental impact and costs in the process of selectively separating rare metals such as cobalt in organic solvents.
Background & Technology
There are only a few ways to improve reaction efficiency in the liquid phase of a substance, especially in guest-host reactions. On the other hand, in an environment such as in a cell, where proteins, nucleic acids, sugars, and other macromolecules are combined at high concentrations, the reaction behaviors of molecules change owing to exclusion volume effects, osmotic pressure effects, and other factors. These conditions are called molecular crowding environments (right side of the figure). A researcher, assistant professor Akihisa Miyagawa, and his colleagues at the University of Tsukuba have been researching a technology that a molecular crowding environment can improve reaction efficiency in engineering chemical reactions.
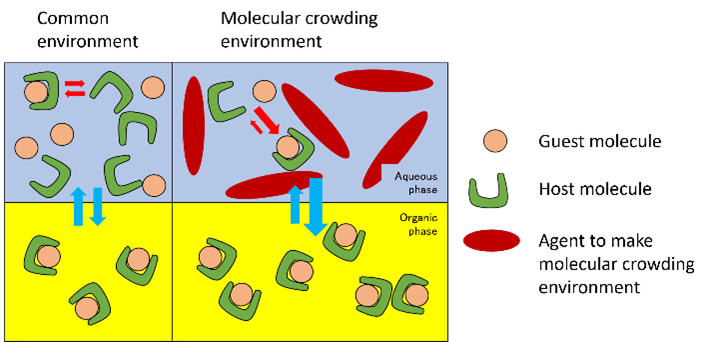 |
As a result of their research, they found a new technique that greatly increases the reaction rate of complex formation reactions. For example, consider the reaction of solvent extraction of a complex consisting of a metal ion (e.g., cobalt ion) in the aqueous phase and a ligand (oxine) in the organic phase. The researchers demonstrated that the addition of a molecular crowding agent (e.g., dextran) to the aqueous phase enhanced the reaction of complex formation in the organic phase and promoted solvent extraction. The following industrial advantages are expected from the application of this technology.
・Streamline the production of various compounds synthesized by host-guest reactions.
・Reducing the amount of organic solvents and improving extraction efficiency when selectively separating rare metals such as cobalt
Researcher
Assistant professor Akihisa Miyagawa (University of Tsukuba)
Patent & Publication
Patent applied in Japan. PCT application is planned.
You will find some publications what related to this technology.
- Miyagawa, H. Komatsu, S. Nagatomo, and K. Nakatani, Effect of Molecular Crowding on Complexation of Metal Ions and 8-Quinolinol-5-Sulfonic Acid., J. Phys. Chem. B 2021, 125, 9853-9859. [Supplementary Cover]
- Miyagawa, H. Komatsu, S. Nagatomo, and K. Nakatani, Acid Dissociation Behavior of 8-Hydroxyquinoline-5-Sulfonic Acid in Molecular Crowding Environment Modeled Using Polyethylene Glycol., J. Mol. Liq. 2022, 360, 119526.
- Miyagawa, K. Nakatani, J-aggregation of 5, 10, 15, 20-tetraphenyl-21H, 23H-porphinetetrasulfonic acid in a molecular crowding environment simulated using dextran., Anal. Sci. 2022, 38, 1505-1512. [Hot Article]
- Miyagawa, H. Komatsu, S. Nagatomo, and K. Nakatani, Thermodynamic Complexation Mechanism of Zinc Ion with 8-Hydroxyquinoline-5-Sulfonic Acid in Molecular Crowding Environment., J. Mol. Liq. 2022, 372, 121181.
Expectation
The University of Tsukuba is seeking companies interested in commercializing this technology. It is expected to improve the yield of host-guest reactions and save costs and lead times. Although research at the university is at an early stage, this technology is promised to help your company’s chemical synthesis business. We can propose joint research that fits your issues. If you decide to adopt this research as a business, we will license the patent held by the university. Please contact us if you are interested in this research.
Project No. DA-04410


