(1) Organoids with villus-like structures in the crypts and including Paneth cells and embryonic cells.
(2) 2D culture system with villus-like structures that can be evaluated in trans-wells
Advantage and Core Benefit
- Drug evaluation is possible with cells that have properties like the living intestinal tract in structure and expression of relevant genes compared to existing intestinal epithelial cells
- Disease-specific iPS cells can be used to develop pathological models
- Organ-on-chip development based on 2D culture system is also possible
Background and Technology
Intestinal organoids are an important tool for the study of intestinal diseases and pharmacokinetics, and iPS cell-derived intestinal organoids are expected to be evaluated close to human biological systems. On the other hand, existing differentiation induction methods require Matrigel-embedded culture, making mass production difficult.
The inventors succeeded in inducing intestinal organoids from human iPS cells by floating culture, which have a crypt villi structure that more closely resembles the structure of the intestinal tract in vivo, and expression of various intestinal markers such as Paneth cells and embryonic cells that are similar to human intestinal tissue. We have also developed a method of induction without using biological materials such as Matrigel for regenerative medicine.
Furthermore, we succeeded in establishing a two-dimensional culture system for the induced organoids using the air-liquid interface culture method. The intestinal barrier function of the cells produced by this two-dimensional culture system was like that of the intestinal tract in vivo, and a crypt villi-like structure was also observed. The iPS cell-derived organoids are expected to be used in a wide range of fields, such as pharmacokinetic evaluation, safety testing, and evaluation of interactions with immune cells, etc., and are expected to be more accurate, closer to the biological system, and easier to use than existing evaluation systems using intestinal epithelial cells.
Data
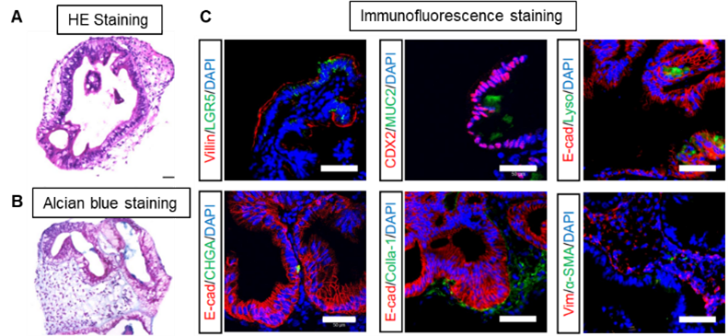
- Morphology (A: HE staining) and secretory function (B: Alcian Blue staining) of intestinal organoids derived from iPS cells and markers of each component cell (intestinal epithelial cells: Villin, goblet cells: MUC2, mid and hindgut: CDX2, stem cells: LGR5, Paneth cells: Lysozyme, endocrine cells: Chromogranin A (CHGA)) (C)
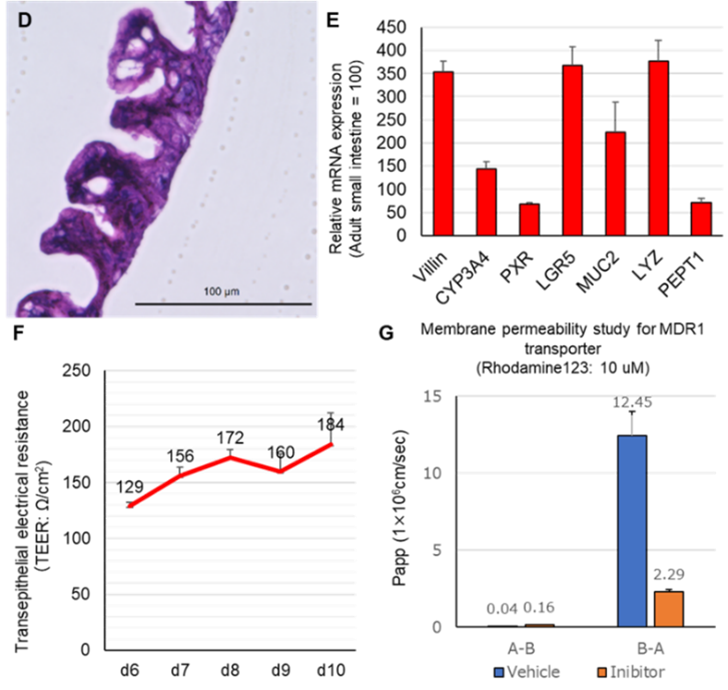
- Intestinal organoids in 2D expanded culture in air-liquid (D: HE staining), expression of markers in constitutive cells (drug metabolizing enzyme-related: CYP3A4 and PXR, LYZ: Lysozyme, peptide transporter: PEPT1) (E), trans epithelial electrical resistance (TEER) values (F), drug (Roadmine123) efflux activity of MDR1 (P-gp) transporter and its inhibitory effect by inhibitors (G)
 |
 |
Patent & Publication
WO/2018/207714, WO/2020/091020
Biomaterials (2022) DOI: 10.1016/j.biomaterials.2022.121696
J Pharm Sci. (2021) DOI: 10.1016/j.xphs.2021.03.014
Researcher
Dr. Tamihide Matsunaga (Nagoya City University)
Expectations
We are seeking to partner with cell product development companies that are interested in licensing this invention to commercialize intestinal organoids and 2D culture test systems. Licenses are also available for experimental research and service applications for pharmaceutical companies and drug discovery support companies.
Project No. WL-02804a


