Advantages
- Higher inhibition than known metal chelator alone inhibitors of metallo-beta-lactamases
- Inhibitory effect on a wide range of metallo-beta-lactamases including NDM-1 and IMP-1
- Low cytotoxicity and acute toxicity in mice
- Novel compounds
Background and Technology
In recent years, reports of infectious disease-causing organisms that have acquired resistance to β-lactam antibiotics have been increasing, and the difficulty of treating these organisms has become an issue. The mechanism of resistance in many cases is the production of β-lactamases that degrade and inactivate β-lactam antibiotics. Metallo-β-lactamases are metalloenzymes that contain zinc in their active center, while serine β-lactamases are enzymes with a serine residue in their active center.
Metallo-β-lactamases exhibit broad substrate specificity, and the bacteria that produce them are a threat because they are resistant to many β-lactam antibiotics. For example, they also degrade carbapenem antibiotics, which are stable against serine beta-lactamases. In addition, metallo-β-lactamases have been identified in many species of bacteria, and multidrug resistance due to metallo-β-lactamase production by Pseudomonas aeruginosa is particularly problematic. Currently, β-lactamase inhibitors used in clinical practice include clavulanic acid, sulbactam, and tazobactam, which are effective in inhibiting serine-β-lactamase, and no inhibitor effective against metallo-β-lactamase has been put to practical use.
We have demonstrated that a new group of compounds, in which a metal chelate structure is introduced into a β-lactam antibacterial agent, shows high inhibition against a wide range of metallo-β-lactamases (NMD-1, IMP-1, etc.) and has a high safety profile.
 |
Reference
- Published patent application: WO2022/239872
Principal Investigator
Tomohiro SAWA Professor (Kumamoto University Faculty of Life Science)
Current Stage and Next Step
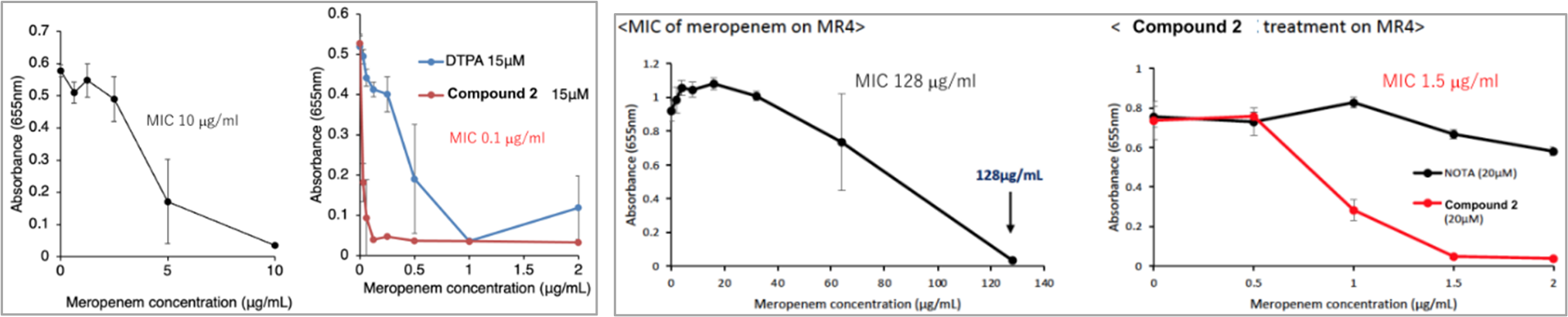
- The compound has been demonstrated to increase the susceptibility of E. coli (clinical isolate) expressing metallo-beta-lactamase IMP-1 to meropenem (carbapenem antibiotic) by more than 100-fold (upper left figure) and multidrug-resistant Pseudomonas aeruginosa (clinical isolate) by more than 85-fold (<2µg/ml) (upper right figure).
- No adverse events have been confirmed in cytotoxicity studies on cultured human cells and acute toxicity studies on mice.
- We plan to investigate the therapeutic efficacy and irreversibility of the inhibitory effect using mouse infection models. We are looking for partners to collaborate with for clinical development.
Project No. BK-04644


