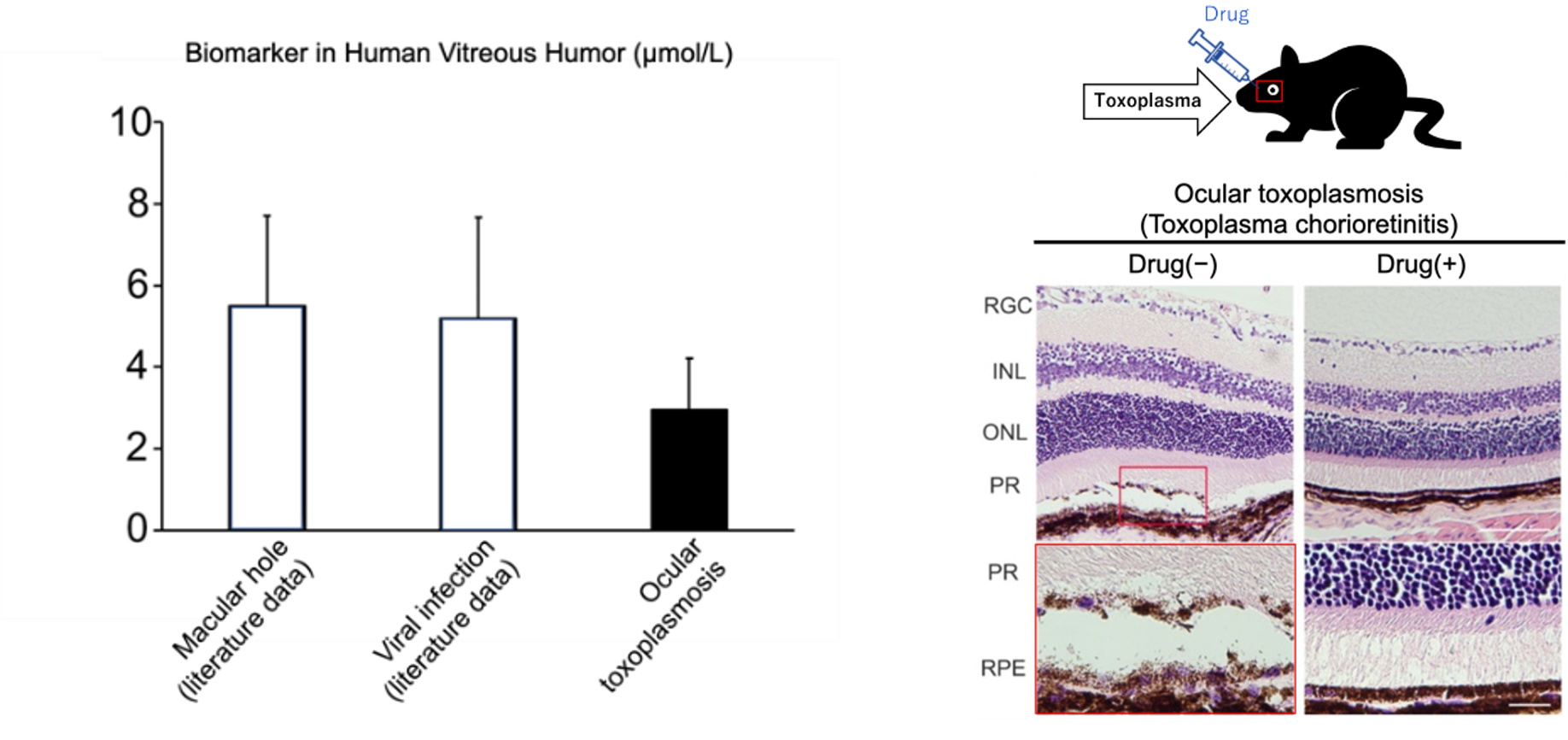
Advantages
- A kit for simple and rapid diagnosis of ocular toxoplasmosis & chorioretinitis.
- Intraocularly administered therapeutic agents (reformulation of existing drugs) selected based on the above diagnosis.
Background and Technology
Toxoplasmosis is a zoonosis caused by infection with the intracellular protozoan parasite Toxoplasma. Toxoplasmosis can be transmitted from undercooked meat or cat feces (acquired toxoplasmosis) or from a pregnant woman to her fetus (congenital toxoplasmosis). Healthy adults have no symptoms (latent infection), but when the immune system is weakened, encephalitis, retinitis, myocarditis, etc. may develop due to toxoplasmosis. In ocular toxoplasmosis (toxoplasmic chorioretinitis), the toxoplasma protozoa enter the eye via the bloodstream, causing severe inflammation and retinal degeneration, resulting in vision loss. Since toxoplasmosis cannot be diagnosed by antibody titers due to the high incidence of latent infection, and the detection rate of toxoplasma gene PCR test of vitreous humor is about 20-30%, if inflammation is seen on fundus examination, antibiotics are administered to determine if they are effective.
Patients are not so common in Japan, but are more common in South America and Africa, especially in South America, where it is reported that 24% of patients with ocular toxoplasmosis have gone legally blind in at least one eye.
 |
Reference and Patent
- Patent pending
Principal Investigator
Hiroki KANEKO (Nagoya University Graduate School of Medicine)
Current Stage and Next Step
- To date, proof of concept has been demonstrated in human specimens and animal studies, and details of the mechanism are under investigation. Also, investigating devices for use in simple rapid tests.
- Seeking collaborative partners to develop simple rapid diagnostic kits and therapeutics; can disclose more information upon signing a CDA.
Project No. BK-04310


