Advantages
- Oral administration induces as high immunity as wild type virus.
- Easy to generate subvariant, and multivalent vaccine.
- Potentially becomes platform technology for combination vaccine.
Background and Technology
Currently two live attenuated rotavirus vaccine, Rotarix and RotaTeq have been commercialized. However, serious side effect intussusception, enfolding of one segment of the intestine within another, sometimes happens, and risks of leaking through feces and acquiring virulence are pointed out. Safe vaccine is awaited, but split vaccines or subunit vaccines are not effective enough.
Reverse genetic (RG) system is useful in terms of research and safe vaccine development and many kinds of RG, including influenza, ebolavirus had been reported.
Recently, researchers from Osaka Univ. have succeeded in establishing complete reverse genetics rotavirus (PNAS, 2017 DOI: 10.1073/pnas.1618424114). Using this technique, they developed single-round infectious rotavirus (SRIR) system composed of virus with incomplete genome set and host cells that express the viral protein. The virus can only proliferate in the specific host cell culture, but not, only viral genes are expressed in normal cells of recipients.
Data
- Certain gene modified rotavirus was well amplified in the gene transgenic culture cells.
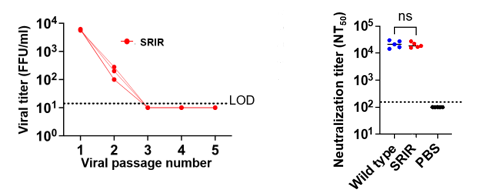
- SRIR did not proliferate in normal culture cells (fig L).
- Blood from vaccine inoculated mice has comparable neutralization ability as infected (fig R).
 |
Patent/Publication
Pending, unpublished
Researcher
Prof. Takeshi Kobayashi, Res. Inst. for Microbial Diseases, Osaka Univ., Japan
Expectations
– We are seeking companies who could license and commercialize this technology.
– Samples can be provided under MTA.
Project No. KJ-01615a


