Advantages
- Novel mechanism of action to enhance cardiac contraction without affecting Ca2+ transient.
- Restores contraction speed and force, promotes sarcomere organization in vitro.
Background and Technology
Catecholamines and PDE3 inhibitors are commonly used for advanced stage of heart failure with reduced ejection fraction (HFrEF) treatment. But there are concerns about worsening prognosis due to side effects (ischemia, arrhythmia, sudden death) caused by an increase in intracellular calcium concentration.
Direct myosin activator omecamtiv mecarbil(OM), which Ca2+ independently enhance myocardial contractility by increasing duty ratio of actin-myosin binding cycle, is being developed. However, result of phase III trial showed that OM did not improve exercise capacity. Heart failure treatments are still highly unmet needs.
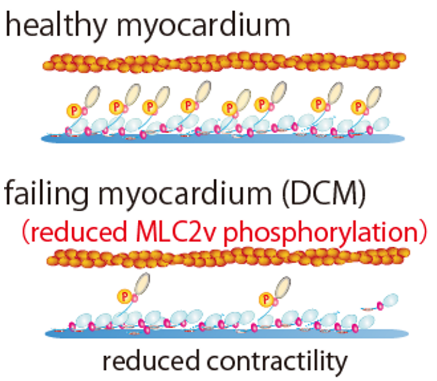
Newly created compound is a allosteric cMLCK activator (Fig. L). Cardiac-specific myosin regulatory light chain kinase (cMLCK) regulates cardiac sarcomere structure and contractility by phosphorylating ventricular isoform of myosin regulatory light chain (MLC2v). The research team has established a high-throughput screening (HTS) system, performed HTS, obtained hit compounds, created derivatives and selected in view of activity, specificity and cytotoxicity.
Data
- LEU-1154 doubled kinase activity of cMLCK.
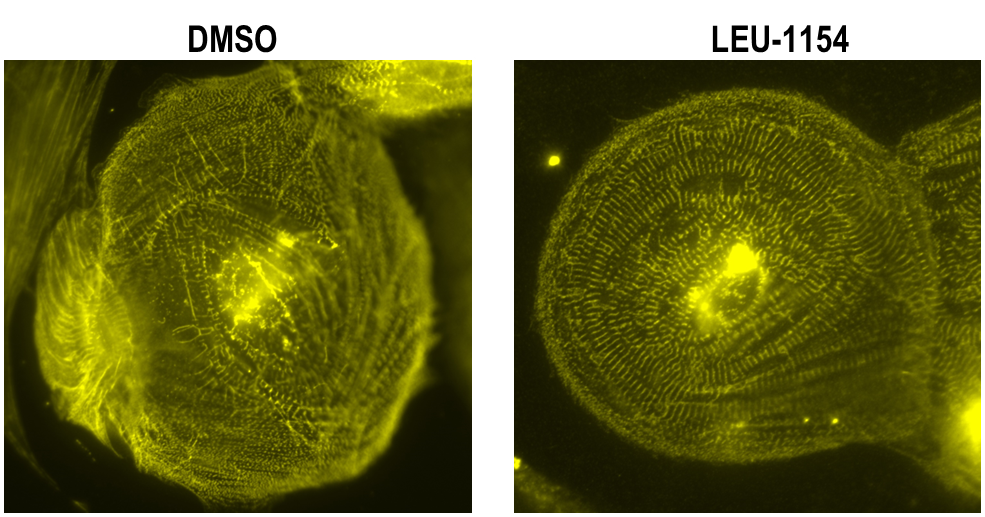
- LEU-1154 increased contraction speed and force, and promoted sarcomere assembly in iPSC-differentiated cardiomyocytes from dilated cardiomyopathy patients with dilated cardiomyopathy (Fig. R), which did not happen by OM.
 |
 |
Patent/Publication
Patent: pending (unpublished)
Publication: Circulation. 2023 May 2. doi:10.1161/CIRCULATIONAHA.122.062885
Researcher
Prof. Osamu Tsukamoto, The University of Osaka and Hyogo Medical University
Expectations
- We are seeking companies who license, succeed from in-vivo study.
- Joint research for optimizing or screening other molecules is welcom
- Details can be disclosed under CDA.
Project No: KJ-04306


