Advantage and Core Benefit
- Screening test for colorectal cancer patients is possible with a simple urine test
- More sensitive than fecal occult blood test, which has a sensitivity of about 70%.
- Excellent screening method for early-stage patients
Background and Technology
The gold standard for colorectal cancer diagnosis is colonoscopy and biopsy. However, due to its invasiveness, cost, and time required for the test, it is not suitable for screening in medical examinations.
Fecal occult blood test is used for primary screening of colorectal cancer in asymptomatic individuals. While it is said to have a sensitivity of more than 70% for patients with stage 2 or later colorectal cancer, it is not a satisfactory test for early detection of colorectal cancer because the sensitivity is estimated to be less than 30% for patients with early stage 0-1 colorectal cancer at most.
The inventors performed ELISA analysis using urine samples from 220 members of the training cohort (110 healthy subjects and 110 colorectal cancer patients) against 15 molecules identified by comprehensive mass spectrometry analysis of urinary proteins from 32 members of the Discovery Cohort (16 healthy subjects and 16 colorectal cancer patients). The analysis revealed significant differences in the expression of four factors including Marker A and Marker B.
Data
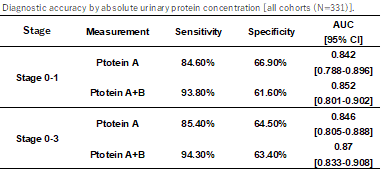 |
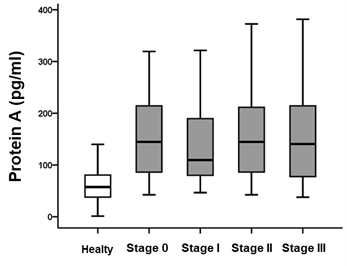 |
- Diagnostic accuracy by urinary protein concentration (left table) and Urinary Marker A Concentrations by Colorectal Cancer Stage (right figure)
- Colorectal cancer can be diagnosed with a sensitivity of 94.3%, specificity of 63.4%, and AUC=0.870, and in the diagnosis of Stage 0-1 early-stage colorectal cancer patients, the accuracy was 93.8% sensitivity, 61.6% specificity, and AUC=0.852.
Patent
Pending in Japan (Unpublished)
Researcher
Dr. Takaya Shimura (Nagoya City University, Gastroenterology)
Expectations
We are seeking a company that can develop a screening test kit for colorectal cancer based on this invention. We are particularly interested in developing kits using methods other than immunochromatography, such as ELISA or latex-based quantitative methods.
Project No: WL-04283


