Advantage and Core Benefit
- New treatment concept based on the mechanism of migration of neural stem cells to the injured area and their differentiation into neurons.
- Expected to be expanded to treatment for brain damage as well as stroke.
- Neurons could be distributed to any part of the brain in regenerative medicine using neural stem cells
Background and Technology
Neural stem cells are abundant in the subventricular zone (V-SVZ) of the brain and actively produce neurons. It is thought that newly generated neurons migrate within the brain and contribute to neural regeneration after brain injury. The inventors have identified N-Cadherin as a factor required for migration of newly generated neurons, which is expected to be used to treat brain injury and cerebral infarction using endogenous stem cells. However, how to use factors such as N-Cadherin to migrate newborn neurons to the site of injury has been a challenge.
RADA4, a self-assembling peptide consisting of 16 amino acids with RADA repeated four times, is known to self-assemble on fibers when injected into the affected area. The inventors developed RADA4-A16G peptide with improved physical properties (mRADA) and synthesized a peptide that adds N-cadherin to mRADA (Ncad-mRADA) to investigate whether the migration of newborn neurons in the brain can be controlled in the Ncad-mRADA injection group. migration distance from the SVZ was predominantly longer and motor dysfunction after brain injury was improved in the Ncad-mRADA-injected group.
Data
 |
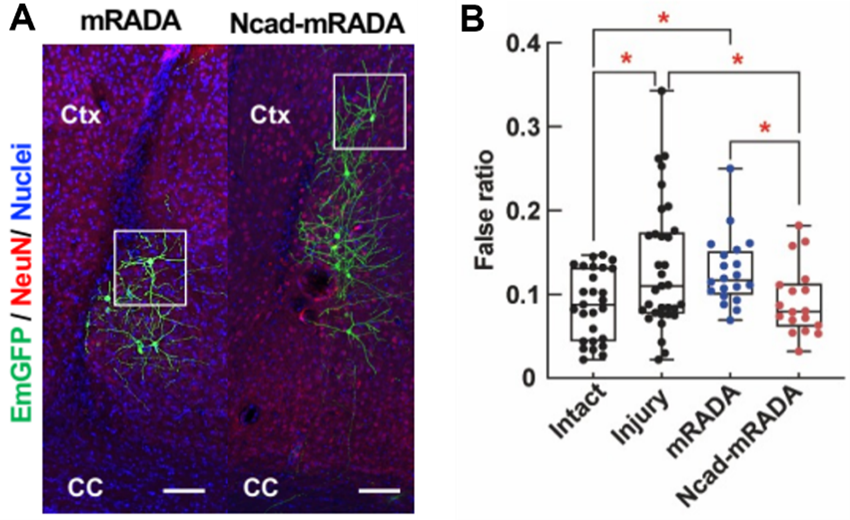 |
V-SVZ-derived cells matured into neurons in both mRADA- and Ncad-mRADA-injected groups, and the Ncad-mRADA-injected group showed significantly longer migration distance from the V-SVZ (A) and a significantly improved increased slip rate, a factor in left hindlimb motor dysfunction (B)
Patent
Patent Pending in Japan
Ohno et. al. Biomaterials (2023) https://doi.org/10.1016/j.biomaterials.2023.122003
Researcher
Dr. Kazunobu Sawamoto (Nagoya City University)
Expectations
We are looking for companies that are interested in developing neurodegenerative medicines based on our Ncad-mRADA peptide technology. We are also open to collaborations with companies that have cellular drug technologies for neurological diseases; paid MTA for evaluation of mRADA peptides is also available.
Project No: WL-04188


