Advantages
- Liquid biopsy can accurately predict response to PARP inhibitor therapy in patients with ovarian cancer.
Background and Technology
Recently, PARP (poly ADP-ribose polymerase) inhibitors have been approved as molecular target drugs for ovarian cancer maintenance therapy. Conventional molecular target drugs include VEGFR inhibitors, but there are no clear drug selection criteria at this time. MyChoiceⓇ has been used as a test to determine response to PARP inhibitor therapy in ovarian cancer patients, but it is not sufficient. MyChoiceⓇ is a method to determine PARP inhibitor responder based on HRD Score from genetic HRD error analysis in BRCA1/2 gene mutations in breast cancer patients.
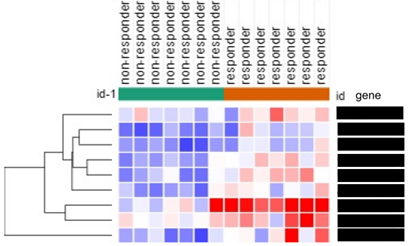
PARP inhibitors are effective in hereditary ovarian cancer, but responder diagnosis is necessary in sporadic cases. Recently, it has been reported that copy number of cancer-related genes is a problem in ovarian cancer. We treated ovarian cancer maintenance therapy patients with PARP inhibitors for about 3 years and analyzed the copy number of cancer-related genes in tissue and fluid exosomes using droplet digital PCR for each responder and non-responder group. As a result, we found that the copy number of specific genes can determine the sensitivity to PARP inhibitors.
We are also studying various other markers and candidate drugs for gynecological diseases, as well as extracellular vesicles.
 |
Reference and Patent
- Patent pending
Principal Investigator
Akira YOKOI (Department of Obstetrics and Gynecology, Nagoya University Graduate School of Medicine, Tokai National Higher Education and Research System)
Current Stage and Next Step
- Currently, we have identified multiple gene copy number markers that predict response to PARP inhibitor therapy for ovarian cancer patients.
- In the future, we plan to further increase the number of cases analyzed to improve the reliability of marker refinement and determination.
- We are looking for pharmaceutical companies to develop PARP inhibitors, droplet digital PCR device companies, and diagnostic service companies as collaborative partners.
Project No: BK-04309


