Core benefit
- Newly identified the release mechanism of SASP factors in the tumor microenvironment.
Background and Technology
SASP, frequently observed in the tissue-resident fibroblasts, is a highly context-dependent phenotype showing both beneficial (e.g., tissue repair) and detrimental roles (e.g., tumor progression) . However, the detailed molecular mechanisms, such as the release mechanism of SASP factors, which can be a molecular target for cancer therapy, and how SASP factors influence tumorigenesis in each biological context have been incompletely understood.
Researcher think the role of SASP in cancer seems varied depending on the stage of cancer, and it has been increasingly recognized to exhibit a tumor promoting role as CAFs (cancer-associated fibroblasts) in the tumor microenvironment of advanced tumors. It may be a tumor-suppressive role of SASP in the very early stage of hepatocarcinogenesis.
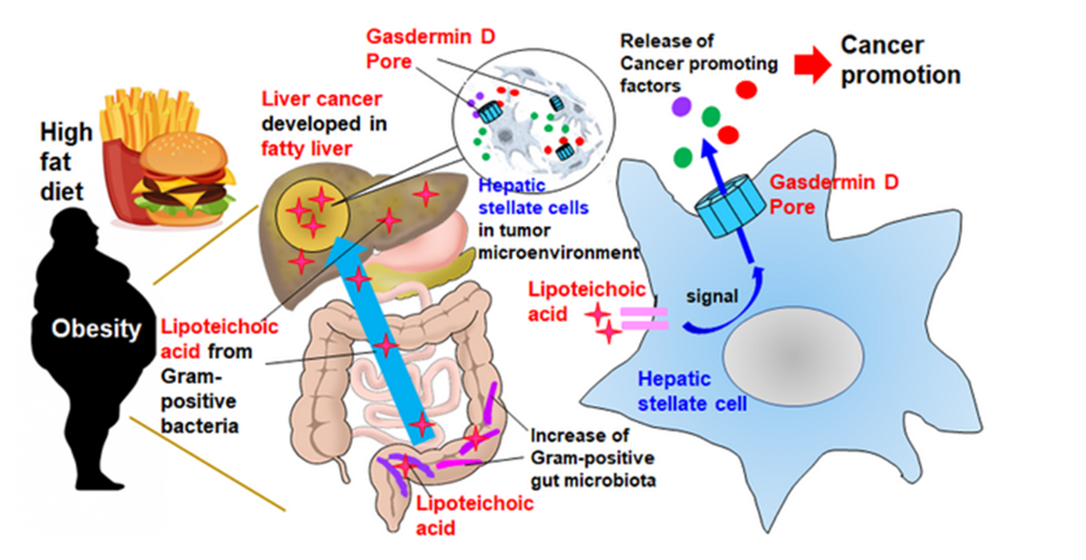
In this study, using a mouse model of obesity-induced hepatocellular carcinoma (HCC) for identification of the release mechanism of SASP factors. High-fat diets weaken gut barrier function, resulting in the translocation and accumulation of lipoteichoic acid in the liver. The accumulated lipoteichoic acid stimulates the cleavage of gasdermin D protein, forming cell membrane pores where the SASP factors, such as IL-1β and IL-33, are released from senescent hepatic stellate cells. IL-33 activates IL-33 receptor-positive regulatory T cells that suppress immune response, accelerating cancer development.
 |
Patent and Publication
- Patent filed, not yet published
- Yamagishi et al., Immunol. 7, eabl7209 (2022)
Principal Investigator
Prof. Naoko Ohtani (Department of Pathophysiology, Graduate School of Medicine, Osaka Metropolitan University)
Current stage and Partnering
- In this study, using a mouse model of obesity-induced hepatocellular carcinoma (HCC) for identification of the release mechanism of SASP factors.
- The GSDMD N-terminal domain was detected specifically in HSCs in human nonviral HCCs, suggesting that a similar release mechanism of SASP factors could be conserved in the HSCs in human nonviral HCC tumors.
- Disulfiram, an inhibitor of pore formation by GSDMD N terminus repressed liver tumor formation in vivo.
We are seeking a partner interested in drug development based on this research result.
Collaboration research regarding screening new compound as GSMND pore inhibitor is welcome.
Repositioning disulfiram as anti-cancer drug is also efficient approach.
Project No: TT-04457


