Advantages
- No theoretical by-products
- Avoids the risk of side effects from by-products
- Eases the purification process in manufacturing
- Equivalent to IgG antibodies in terms of properties and stability
Background and Technology
Bispecific antibodies (BsAbs) are a sort of dual functional proteins with specific binding to two distinct targets, which have become a focus of interest in antibody engineering and drug development research and have a promising future for wide applications in cancer immunotherapy and autoimmune disease. In particular, IgG-type BsAbs exhibit superior advantages in structure (similar to natural antibodies), pharmacokinetics, half-life, and biological activity.
IgG-type BsAbs typically require four different polypeptide chains (two types of heavy chains and two types of light chains), that when mismatched may produce a variety of by-products. The problem of chain mismatches will reduce the yield of the BsAb, increase the difficulty of purification, and increase the production cost. Therefore, obtaining a correctly assembled BsAb has become a key issue. In the past few decades, many strategies have been developed to improve the homogeneity of the product and the yield of the desired BsAb, but by-products are still generated. In addition, the by-products may cause unexpected activation of immune cells, leading to the risk of side effects.
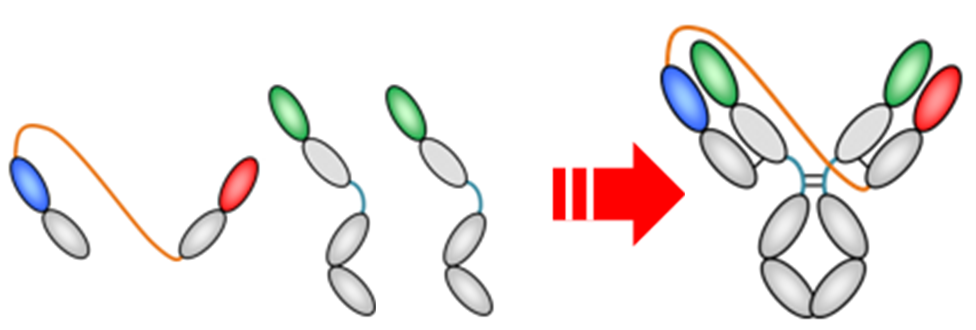
This technology, named TribsMab CLC ‒ Trimeric bispecific Monoclonal antibody Common Light Chain, is composed of three polypeptide chains of two types, as shown in the figure below. One type of polypeptide chain has a structure in which two heavy chain variable regions with constant regions are linked by a peptide linker. The other type has a common light chain. TribsMab CLC yields no theoretical by-products without Fc heterodimerization.
 |
Patent
PCT/JP2021/012911 (pending in US and JP)
Principal Investigator
Associate Prof. Takeshi Nakanishi (Division of Science and Engineering for Materials, Chemistry and Biology, Graduate School of Engineering, Osaka Metropolitan University)
Current stage and Partnering
- Researchers generated HER2/HER3, CD20/CD3, and BCMA/CD3 TribsMab CLC. These TribsMab CLC antibodies showed binding activity and inhibitory activity in vitro assay.
- In vivo validation has not yet been demonstrated and will need in the future.
- If you use TrisMab CLC for your desired target, please let us know.
Seeking a biotech/pharmaceutical company to focus on antibody drug development as a development partner on this project.
Project No: TT-04346


