Advantage and Core Benefit
- Repositioning of patent-expired safety-proven drug reduces development cost and time.
- Theoretical effective dose, 2mg of losartan for 50kg patient much smaller than commercialized dosages avoids risk of hypotensive side-effect, as well as off-label use.
- Potentially multiple indications: liver, kidney, lung, etc. with novel mechanism of action.
Background and Technology
Angiotensin receptor blockers (ARBs) have been reported to be beneficial for liver fibrosis, but its mechanisms remain unclear. Also, ARB is contraindicated for patients with high risk of hypotension.
Our researcher found that even ultralow dose ARB can alleviate liver fibrosis through mechano-transduction regulation. 0.5 mg/kg/day, theoretically equivalent to 0.04 mg/kg/day for human, Losartan administration interferes the expression of several mechano-transduction related molecules, and effectively alleviates liver fibrosis of model mice.
This technology is potentially appliable for fibrosis of kidney, heart lung, stomach, intestine, skeletal muscles, as well as skin.
Data
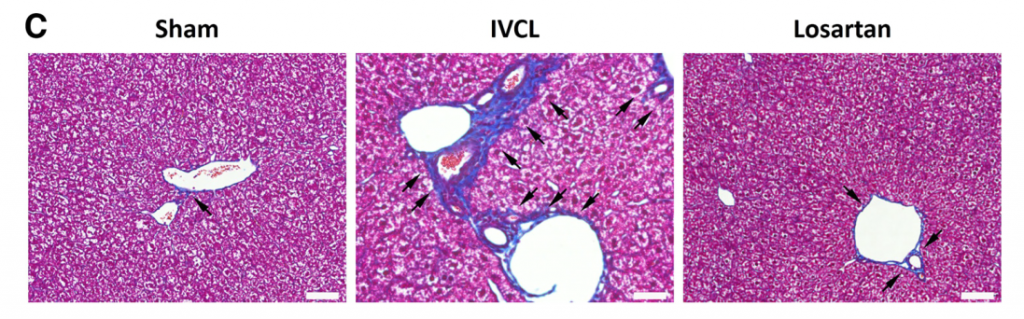
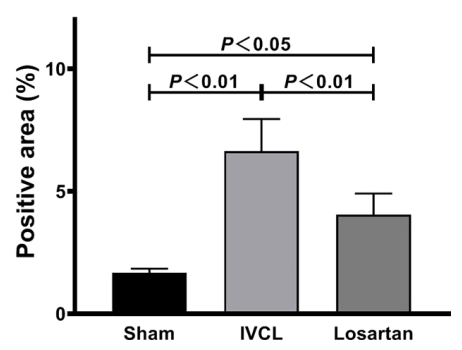
- Hydrostatic pressure-induced liver fibrosis mouse model was established by partial inferior vena cava ligation (IVCL). 8 week, 0.5 mg/kg/day losartan treatment reduced fibrosis (fig), attenuated upregulation of alpha-SMA, mechano-transduction related molecules; RhoA, ROCK1, p-MLC2.
- 50 mmHg hydrostatic pressure imposed human hepatic stellate cell ex-vivo model confirmed effect of 10nM losartan for mechano-transduction response.
- Effect also confirmed in unilateral ureteral occlusion kidney fibrosis model.
 |
 |
Patent and Publications
Patent: WO/2023/140350, pending in JP, US, EP.
Journal: American Journal of Physiology-Gastrointestinal and Liver Physiology, Vol. 322, No. 4 https://journals.physiology.org/doi/abs/10.1152/ajpgi.00238.2021
Researcher
Prof. Tao-Sheng Li, Department of Stem Cell Biology, Atomic Bomb Disease Institute, Nagasaki University, Japan
Expectations
We are seeking a partner who will commercialize low dose ARBs as anti-fibrosis drug.
The researcher is welcome for joint research for specific fibrotic disease.
Project No.KJ-04320


