Background and Technology
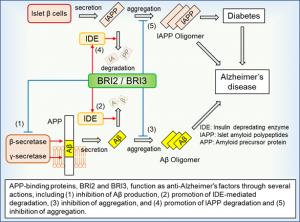
BRI2/BRI3 is a molecule that binds to amyloid precursor protein (APP) and is known to promote the degradation and inhibit the aggregation of amyloid-β (Aβ). Aβ is secreted when APP is cleaved by β and γ-secretase, and BRI2/BRI3 inhibit this secretion pathway of Aβ by binding to APP. The systemic expression of BRI2 is also involved in the inhibition of aggregation of islet amyloid polypeptide IAPP, which is a major component of amyloid deposition in type 2 diabetic patients (upper panel). In practice, genetic mutations in BRI2 cause British familial dementia and Danish familial dementia, and therefore, loss of function of BRI2/BRI3 has been associated with the development of amyloidosis.
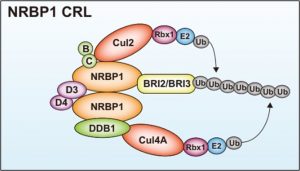
Functional activation of BRI2/BRI3 is expected to be a new target for the treatment of Alzheimer’s disease, but the mechanism of its intracellular regulation has been unclear. Inventors have shown for the first time that the NRBP1-mediated ubiquitin-proteasome system is involved in BRI2 and BRI3 degradation (bottom panel). They have constructed an HTS system for amyloidosis treatment based on the concept of inhibiting the binding of NRBP1 and BRI2, and found that the hit compounds in the initial screening inhibit the production and aggregation of amyloid-β. On the other hand, the toxicity and other properties of this hit compound are not sufficient, and the inventors hope to conduct further screening to obtain more hit compounds.
 |
 |
Data
- Western blotting confirmed that the addition of the hit compound obtained by HTS increased the level of endogenous BRI2 expression and suppressed the ubiquitination of BRI2.
- The addition of this compound decreased the expression levels of Aβ40 and Aβ42 relative to DMSO.
Patent and Publication
- JP 2020-183905
- Yasukawa et al., 2020, Cell Reports 30, 3478–3491
https://www.sciencedirect.com/science/article/pii/S2211124720302278
Researcher
Dr. Teijiro Aso and Dr. Takashi Yasukawa (Koch University)
Current Stage and Expectations
Elucidation of NRBP1-mediated degradation pathway of BRI2/BRI3, a factor that promotes degradation and inhibits aggregation of amyloid-β
Construct a high-throughput screening (HTS) system for inhibitors of NRBP1 binding to BRI2/BRI3.
To obtain hit compounds by collaborating with companies that have compound libraries
Product No. WL-04155


