Advantage and Core Benefit
- Anti-human netrin-4 mouse antibodies cross-react with non-human netrin-4.
- Nerve root injection, commonly used in pain clinic, with small dose applicable.
Background and Technology
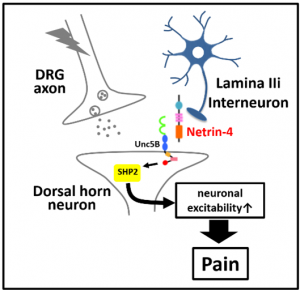
Our researcher has revealed that neuropathic pain signaling is mediated by Netrin-4 and its receptor Unc5B, and demonstrated that blockade of Netrin-4-Unc5B signals by siRNA or polyclonal antibody intrathecal administration can alleviate the neuropathic pain in 2016 (fig. L, publications).
Now, he identified the epitope of Netrin-4, and obtained monoclonal antibodies by mouse immunization with peptide whose sequence is common in human and rodent. Dorsal root administration experiment showed efficacy, which indicated the mAb could make a medicine with novel mechanism for patients with hyperalgesia or allodynia after nerve injury.
Data
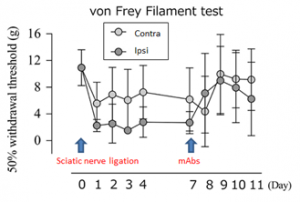
- In rat von Frey Filament test model, 3μg anti-netrin-4 mAb injection into dorsal root 7days after partial sciatic nerve ligation elevated the withdrawal threshold to the contra-lateral level (Fig. R).
- Obtained humanized mAb and confirmed effect by systemic administration (not published).
 |
 |
Patent, Publications
- Background Patent: WO2015/025770, registered in US/EP/JP
- Monoclonal antibody patent: WO2023/190496 applied in US/EP/JP.
- Journal: https://doi.org/10.1073/pnas.1402095111, https://doi.org/10.1084/jem.20160877
Researcher
Prof. Toshihide YAMASHITA (Osaka Univ. JAPAN)
Expectations
We are seeking a partner who will in-license and develop the technology further by humanization of mAbs. Details can be disclosed under CDA, mAbs for review can be provided under MTA (paid).
Project No. KJ-04225


