Advantage and Core Benefit
- Low risk of side effects due to the ability to reduce IL-17 production from CD96-expressing gamma-delta T cells and CD4+ T cells locally without systemic suppression of IL-17
- Potential application in autoimmune diseases associated with IL-17 such as psoriasis, rheumatoid arthritis, atopic dermatitis and multiple sclerosis
Background and Technology
Psoriasis is a chronic inflammatory keratosis of unknown etiology characterized by erythematous plaques and silvery white scales on the skin and affects an estimated 125 million people worldwide. The involvement of IL-17 in the pathogenesis of psoriasis is well known, and antibodies against IL-17 and its receptors have been used as therapeutic agents. On the other hand, since IL-17 is an important cytokine in the defense against infection, the administration of IL-17 inhibitor antibodies has been associated with side effects such as severe infections and exacerbation of inflammatory bowel disease.
Dr. Shibuya’s group identified that the expression of CD155, a ligand for CD96, was up-regulated in γδ T cells in psoriasis. Furthermore, they found that γδ T cells produce IL-17 via CD96 signaling in the pathogenesis of psoriasis. Neutralizing antibodies to CD96 were administered to animal models of psoriasis, which improved the condition of erythema, scaling and skin thickness. Initial data suggest that the therapeutic effect in psoriasis models is comparable to that of anti-IL-17 antibodies, and a therapeutic effect has also been observed in EAE models. We have also confirmed the expression of CD96 on human CD4+ T cells and γδ T cells, and an increase in IL-17 production upon stimulation with CD96 agonist antibodies.
Data
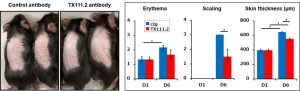 |
- Mice treated with imiquimod on Day 0 were intraperitoneally injected (100 µg/Body) with anti-CD96 antibody (TX111.2) and evaluated the pathological score on Day 5.
- Therapeutic efficacy of TX111.2 antibody against psoriasis was comparable to that of anti-IL-17 and anti-TNF antibodies and showed synergistic effects with TNF antibodies
Patent
Patent No. PCT/JP2020/39693
Researcher
Dr. Kazuko Shibuya, Dr. Kyoko Hayashi and Dr. Akira Shibuya (Tsukuba University)
Expectations
We are looking for companies interested in developing therapeutics with CD96 antibodies for IL-17-related autoimmune diseases such as psoriasis and multiple sclerosis. We have established new anti-human CD96 antibody clones and some antibodies exhibited affinity more 10 times stronger than existing antibodies and be able to create therapeutic humanized antibody based upon this CDR information shortly. We are looking forward collaboration partner for discovery and development of novel humanized anti-CD96 antibody.
Product No. WL-03844


