Advantage and Core Benefit
- It is expected to develop novel therapeutic agents for steroid-resistant, IgE-independent, and unexplained dermatitis.
- A novel therapeutic agent based on the pathogenic mechanism of allergic asthma and atopic dermatitis caused by mites.
Background and Technology
House dust mites (HDMs) are allergens associated with various diseases such as asthma and atopic dermatitis (AD), and about 80% of the causes of AD are said to be HDMs.
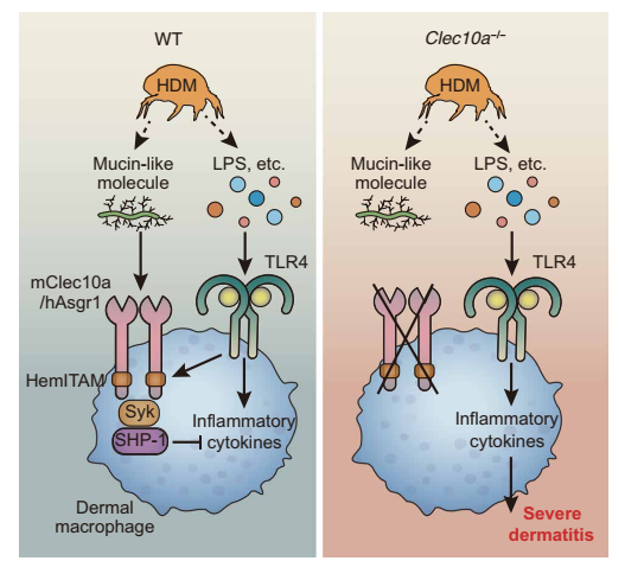
The inventors identified a mutation in the C-type lectin receptor, Clec10a, as the cause of severe AD in response to HDM in NC/Nga mice and clarified the mechanism of HDM-mediated dermatitis (Figure). HDM-induced dermatitis is caused by TLR4-mediated inflammatory response in macrophages. In wild-type mice, mucin-like glycoprotein antigen contained in HDM suppressed inflammation by transducing signals as Clec10a ligand (Clec10a-L), and Asialoglycoprotein receptor 1 (Asgr1) functioned as a homologue of Clec10a in humans. Furthermore, we found that the application of the identified Clec10a-L ameliorated TLR4-dependent dermatitis induced by LPS.
 |
Data
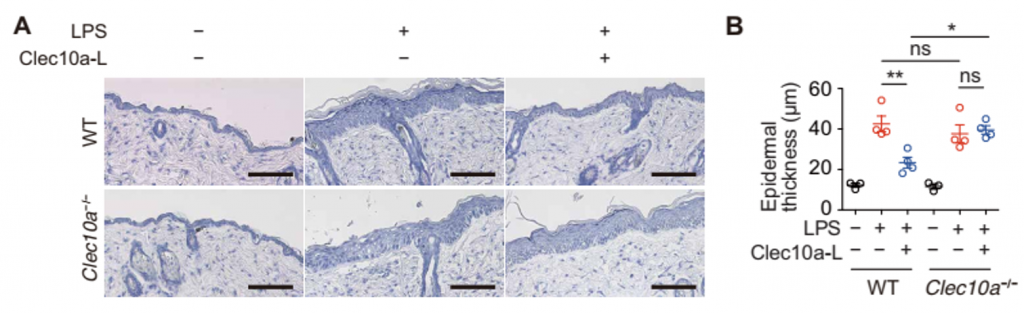 |
- HDM-derived Clec10a-L ameliorates LPS-induced dermatitis. LPS with or without Clec10a-L was applied every day to the dorsal skin of C57BL/6J-WT and Clec10a-/- mice. Histology (H&E: A) and epidermal thickness (B) on day 5 were compared between WT and Clec10a-/- mice.
Patent
Patent No. WO2020/179700
Researcher
Dr. Akira Shibuya(University of Tsukuba)
Expectations
We hope to collaborate with companies that develop therapeutic drugs for allergic diseases based on the glycan antigens identified in this research. We would like to develop optimal modalities for therapeutic drugs through joint research with companies and propose collaboration at the University of Tsukuba for screening and evaluation of drug efficacy based on the function of human Asgr1.
Product No. WL-04059


