Advantage and Core Benefit
- Mechanism of intestinal tissue regeneration by direct administration of intestinal organoids
- Intestinal organoids are not viable, but are desorbed and expelled, resulting in a low risk of undifferentiated iPS cell tumorigenesis.
Background and Technology
Intestinal organoids are an important tool for the study of intestinal diseases and pharmacokinetics, and human iPS cell-derived intestinal organoids are expected to be developed for regenerative and cellular medicine. On the other hand, existing differentiation induction methods from iPS cells cannot reflect the characteristic villi-crypt structure of intestinal organoids. In addition, the culture of Matrigel-embedded cells is difficult to produce in large quantities, which poses a problem for therapeutic use.
By devising the culture conditions during differentiation induction, the inventors have developed a method to produce a large number of intestinal organoids with a villi-crypt structure similar to that of living intestinal structures from human iPS cells without using biological materials such as Matrigel. In addition, by adding low-molecular-weight compounds, we succeeded in inducing intestinal organoids whose expression of various intestinal cell markers, such as intestinal stem cells, Paneth cells and goblet cells, is close to that of human intestinal tissue. Furthermore, when human iPS cell-derived intestinal organoids were administered directly into the intestines of mice with DSS enteritis model, therapeutic effects were observed. Since the administered intestinal organoids were not found to be viable in the intestine, and were thought to be desorbed and expelled, it is indicated that the intestinal organoids had an effect of promoting tissue regeneration of the intestine (trophic effect). It is expected to become a new therapeutic concept for iPS cell therapy.
Data
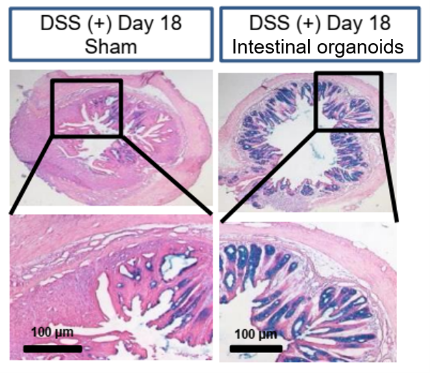
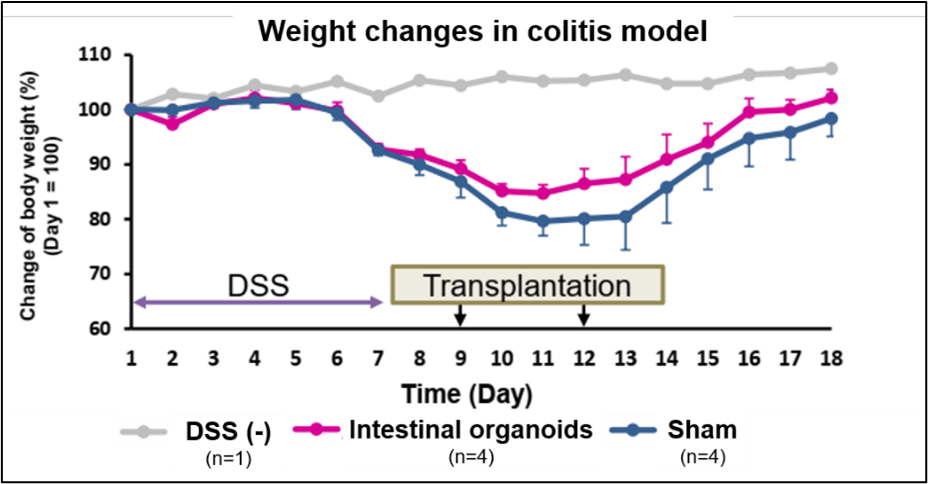
- Mice injected with iPS cell-derived intestinal organoid cell suspension on days 9 and 12 after 1 week of DSS-induced enteritis showed weight recovery and increased mucopolysaccharide production in intestinal tissues.
- Tight junction formation and expression of cell proliferation markers were observed in the rectal tissue of DSS enteritis model mice injected with intestinal organoids.
- No organoid biogenesis was observed.
 |
 |
Patent
Patent No. PCT/JP2018/017572
Researcher
Dr. Tamihide Matsunaga (Nagoya City University)
Expectations
We are seeking a company that develops cell medicine for intestinal diseases based on this technology.
Product No:WL-02804


