Advantages
- Once-daily oral administration keeps high blood concentration for 24h.
- Potential application for NASH/NAFLD related liver dysfunction as well as hyperammonemia, hepatic encephalopathy.
Background and Technology
Ornithine is known as a key amino acid on ammonia metabolism, and L-ornithine L-asparatate complex (LOLA: Hepa-Merz) is used as supplement for improving liver function, and as therapeutics for hyperammonemia that causes hepatic encephalopathy in Europe. On the other hand, ornithine is metabolized quickly and is low bioavailability by oral administration. The researcher synthesized the self-assembling ornithine nanoparticles by formulating poly block co-polymer of poly-ornithine and polyethylene glycol (PEG). Orally administered ornithine nanoparticles accumulate in intestinal mucosa and gradually release ornithine into the bloodstream over 24 hours.
The ornithine nanoparticles can suppress liver dysfunction and reduce blood ammonia concentration, and maintain liver function. Arginine nanoparticles were also synthesized. Arginine replacement is considered to be effective for treatment of ureacycle disorders and cancers.
Hyperammonemia, which is deteriorating factor of non-alcoholic fatty disease (NAFLD) causes hepatic encephalopathy.
Data
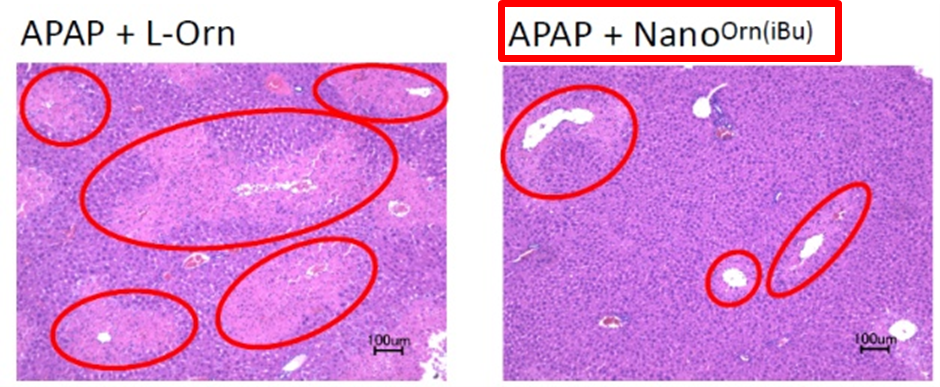
Therapeutic effects were examined in APAP (acetaminophen) induced acute liver injury mouse model. Once daily orally administered nanoparticles with 200mg/kg ornithine, NOT ornithine, reduced blood ammonia concentration(fig. R), hepatocyte necrosis(fig. L), and improved hepatic function (ALT, AST, etc.). Nanoparticles significantly improved survival as compared to L-ornithine.
Arginine nanoparticles have significantly reduced triglyceride level in mouse NASH model.
 |
 |
◆Nanoorn(iBu) :
isobutyryl group-hydrophobized poly-L-ornithine PEG block copolymer self- assembly.
Patent
Pending (WO2022/102608A1) (US, EP, CN, JP)
Researcher
Professor Yukio Nagasaki, Faculty of Pure and Applied Sciences, University of Tsukuba
Expectations
– We are seeking companies to license and commercialize this technology.
– Samples can be provided under MTA.
Project No:KJ-03824


