Advantages
- Scalable and stable in vitro model reflecting hepatocyte metabolic functions for long term
- High reproducibility with connection site between hepatocyte and bile duct
- Monitoring available for metabolism or hepatotoxicity of potential drugs
- Human hepatocytes can be introduced to this hepatobiliary organoid
Background and Technology
Large number of healthy hepatocytes for hepatotoxicity testing in vitro can be isolated from a single rat or from a human liver resection or autopsy. However, primary hepatocytes reduce in function after differentiation, are limited to obtain and have lot-to-lot variation. To avoid these issues, many in vitro hepatotoxicity tests have been conducted using established human hepatoma cell lines such as HepG2 and HepaRG or stem cells derived from liver. But so far, few approaches have succeeded in reproducing a liver organoid with hepatobiliary function. Inventors have succeeded in efficient hepatobiliary organoid from rat/mouse small hepatocyte (SH) and mouse biliary epithelial cell (BEC) and established by the following protocol. Furthermore, the inventors have progressed with hepatocyte derived from Human chemically induced liver progenitors (hCLiP) and structured hepatobiliary organoid model.
<Protocol>
1. Type I collagen solution will be treated and turned into a gel
2. EpCAM(+) BEC cells will be seeded and cultured till the concentration reaches to a certain level
3. hCLiP will be added to the BEC and be cultured
4. Matrigel and Type I collagen will be deposited layers again
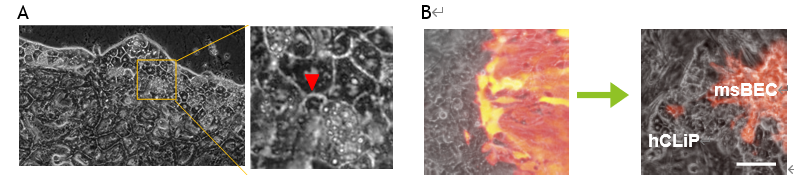
Data
- By using the protocol above, connection site by bile canaliculi have been formed between hepatocyte and bile duct.(A) Co-cultured hCLiP and msBEC structured the connecting surface.(B)
Patent
Pending ; not published yet
Researcher
Dr. Naoki Tanimizu, et al. (Sapporo Medical University, Japan)
Expectations
We are looking for a CRO or a pharmaceutical company to develop and use this hepatocyte model for in vitro hepatotoxicity testing.
Product No. : ON-03088