Advantages
- Improvement of L-DOPA retention in blood
- High effect for motor symptoms of Parkinson’s disease despite low dose
- L-DOPA-induced dyskinesia is avoidable
Update info.
Oral administration efficacy for Parkinson’s motor symptoms of this compound was detected, and low AUC of L-DOPA was also solved.
Background and Technology
Parkinson’s disease (PD) is a movement disorder caused by decrease dopamine level. PD symptom proceeds gradually and gets worse over time. Although some therapies for PD have been developed, there are no radical treatments. Levodopa (L-DOPA), precursor of dopamine is an effective drug for treating motor symptoms of PD. However, sustaining high blood concentration level of L-DOPA is difficult because of poor bioavailability. Additionally, advanced PD is difficult to control, so high dose of the drug is required in late stage PD and it unfortunately induces side effects such as dyskinesia.
This new technology is a block polymer of PEG and L-DOPA. The polymer self-assembles and protect L-DOPA from biodegradation. Therefore, this enables the L-DOPA retention in blood in therapeutic range for treating PD motor symptoms and suppressing the side effect of L-DOPA.
Data
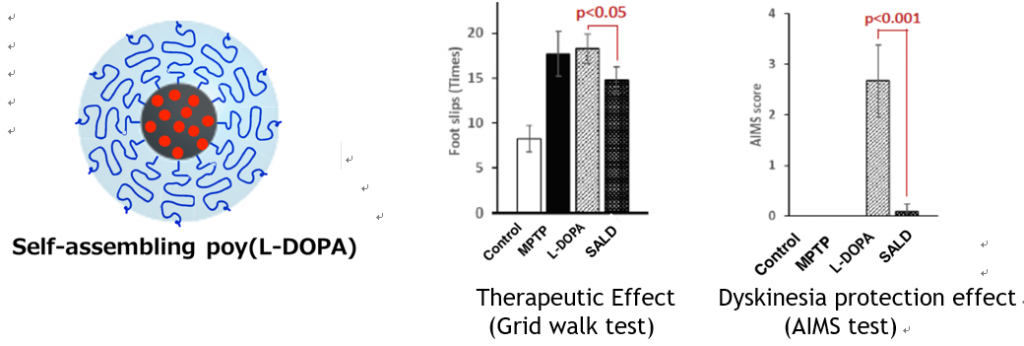
- The therapeutic effect and dyskinesia protection effect of Self Asembling L-DOPA (SALD) was evaluated in PD model mouse adjusted with MPTP.
- SaLD reduced motor symptom expressed from the foot slips in comparison to L-DOPA.
- SaLD suppressed the L-DOPA induced dyskinesia.
Patent/Publications
- WO/2020/166473 Filed in US, EP, JP
- Acta Biomaterialia, Vol 109, June 2020, 220-228
https://doi.org/10.1016/j.actbio.2020.03.021
Researcher
Professor Yukio Nagasaki, Faculty of Pure and Applied Sciences, University of Tsukuba
Expectations
- We are seeking companies to license and commercialize this technology.
- Samples can be provided under MTA.
Product No:KJ-03032