Advantage and Core Benefit
- Periostin (PN) splicing variant specific mAbs, effective and less harmful, has been obtained.
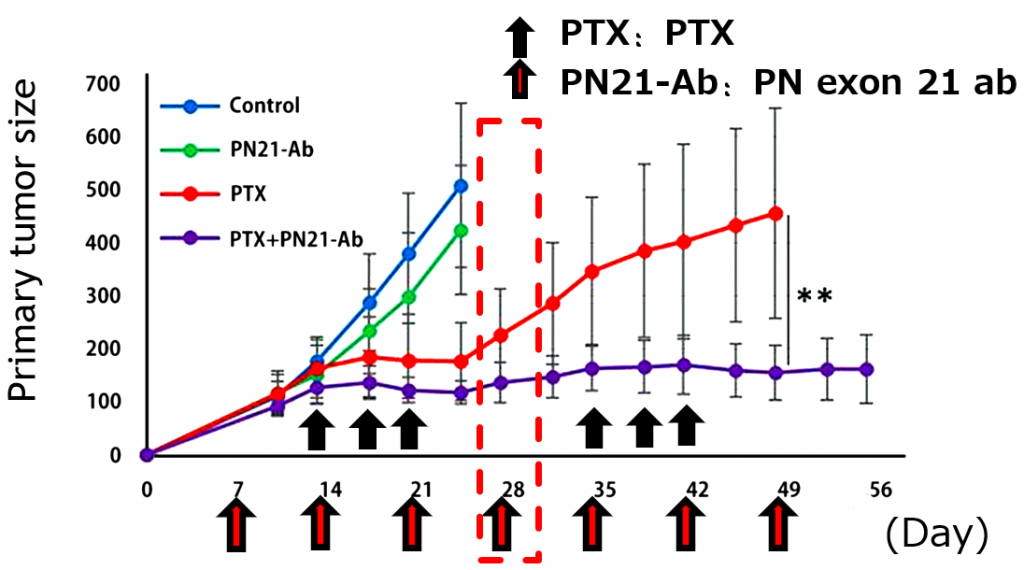
- Excellent therapeutic effect for triple-negative breast cancer(TNBC) xenograft model (Fig).
- RCB for humanized Ab was completed and GLP preclinical study will be started at 2021/3.
- Many other potential indications; glioblastoma, severe asthma, heart failure, diabetic retinopathy, chronic kidney disease.
Background and Technology
Recent reports have indicated that Periostin (PN), an extra cellular matrix protein is secreted by cancer-associated fibroblast [J Pathol. 2015 Feb; 235(3): 466–477.] and plays a crucial role in cancer metastasis by activating Wnt signaling of cancer stem cells [Nature. 2011 Dec 7;481(7379):85-9]. However, few successful study targeting PN had been reported, and some researchers have warned that inhibition of PN is harmful.
The researcher has found that exon (ex) 21-containing PN variants increase after chemotherapy in a mouse model transplanted with human TNBC, and in clinical samples of TNBC. Based on the findings they have created the ex 21 specific antibodies and demonstrated the therapeutic effect (https://www.nature.com/articles/s41598-018-22340-7).
Periostin is involved with many other severe diseases, such as chronic heart failure after acute myocardial infarction, asthma, CKD. They have obtained ex 17 mAbs and demonstrated therapeutic effect against some of them.
Data
- PN ex21 mabs with or without pacritaxicel inhibited growth of primary as well as metastatic TNBC tumors in vivo.
- PN ex21 mabs with pacritaxicel and anti-VEGF Ab or anti-PD-L1 Ab synergistically inhibited TNBC growth and metastasis.
 |
Patents
PCT/JP2006/326280 (JP, US, GB, FR, DE, AU, KR),
PCT/JP2008/061768 (JP, US, EP, CA, CN, KR),
PCT/JP2014/055861 (JP, US, EP)
Researcher
Guest prof. Yoshiaki Taniyama, Graduate School of Medicine, The University of Osaka, Japan
(http://www.cgt.med.osaka-u.ac.jp/cont/e_gr01_a.html)
Expectations
Start-up company Periotherapia (https://periotherapia.co.jp/index.html) is looking for investment
partners in series C round for preparation of clinical trial, as well as co-development companies.
Full experimental data and development status can be disclosed under CDA.


