Advantage and Core Benefit
- CHRNB2 was identified from 300 stage III gastric cancer patient’s resected specimens.
- Potentially effective for other tumors; lung, colorectal, breast, pancreatic cancer, etc.
- Companion diagnostic can be used; expression confirmed in patients’ tissues.
Background and Technology
Gastric cancer (GC) is the second leading cause of cancer-related deaths worldwide. Limited treatment options such as antibodies targeting HER2 and immuno-checkpoint inhibitors are currently available for advanced gastric cancer, whose response rates remain unsatisfactory. Thus, novel anti-cancer agents with different mechanisms are indispensable for improvement of treatment outcomes of patients with GC.
Transmembrane Receptor, cholinergic receptor nicotinic beta 2 subunit (CHRNB2) has been identified via comprehensive transcriptome analysis of 300 resected tissues from stage III GC patients. CHRNB2 was significantly expressed in specimens of patients with poor prognosis compared with five-year survivors without recurrence.
Data
- Intraperitoneal administration of monoclonal antibodies against CHRNB2 significantly reduced tumor growth of human GC peritoneal dissemination model mice (fig).
- Patient’s cancer tissues were stained by Immunohistochemistry.
- Retrospective analysis by quantitative PCR data showed high predictive values to identify patients at risk of recurrences and poor prognosis.
- Tissue microarray data demonstrated cancer-specific overexpression of CHRNB2 in breast, lung, pancreatic, esophageal and colon cancer.
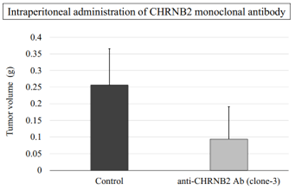 |
Patent, Publications
PCT/JP2019/002504 (WO/2019/146759) US, EP, CN, JP
Oncogene 40, 5495–5504 (2021).
Researcher
Dr. Mitsuro kanda Department of Gastroenterological Surgery, Nagoya University Graduate School of Medicine, Japan.
Expectations
We are seeking a biotech company who will be interested in developing the technology. The researcher is working with a CRO to obtain human mAbs.
Product No:KJ-02408


